Department of Chemistry and Materials Science | Aalto University (external link)
World-class materials research

Hydrogen plays a major role in industrial production. Among its uses are removing sulphur from petrol and diesel, and as a reducing agent in the steel manufacturing industry. While hydrogen itself is a promising energy source for a sustainable future energy system, it has traditionally been created using fossil fuels. As the costs for CO2 emissions continue to rise for industrial producers, there has been greater interest in developing green hydrogen production technologies among the research and production spheres. Refineries and the steel industry are two sectors keen to embrace green hydrogen.
Storing hydrogen is a tricky business. While hydrogen has some properties which are beneficial to storage, such as being very lightweight, allowing larger volumes to be stored and needing less space for this than batteries. However, hydrogen is an incredibly small molecule which is prone to escaping containment over time. Hydrogen storage solutions vary depending on the usage scenario, for example, if it is used directly as a fuel source or stored and converted to energy through fuel cells. The duration of storage and concentration of hydrogen also impacts the metal pipes and containers, which is why efficient hydrogen storage solutions are essential.
Aalto University’s School of Chemical Engineering is a member of FinH2, a cooperative Finnish project looking to produce more efficient and alternative electrolyser solutions and hydrogen technologies with the aim of improving Finland’s competitiveness in the hydrogen sphere. Current electrical energy conversion devices require critical metals, such as platinum, to operate. However, there is a finite amount of these metals in the Earth’s crust. Aalto’s researchers are investigating ways of improving the material and energy efficiency of different electrochemical energy conversion devices. The aim is to drastically reduce the volume of critical metals, or replace them altogether, and to make sure these devices function for as long as possible. Drastically reducing or eradicating mining and recycling requirements has the additional benefit of lowering carbon emissions.
Alternative electrolyser technologies, without critical metals, currently exist. However, these are less efficient than available technologies and emerging electrolysers which use a fraction of the current amount of platinum. This poses an important societal choice – are we willing to accept lower efficiency in exchange for preserving critical metals? These are the sorts of questions we must be willing to face up to in the energy transition.
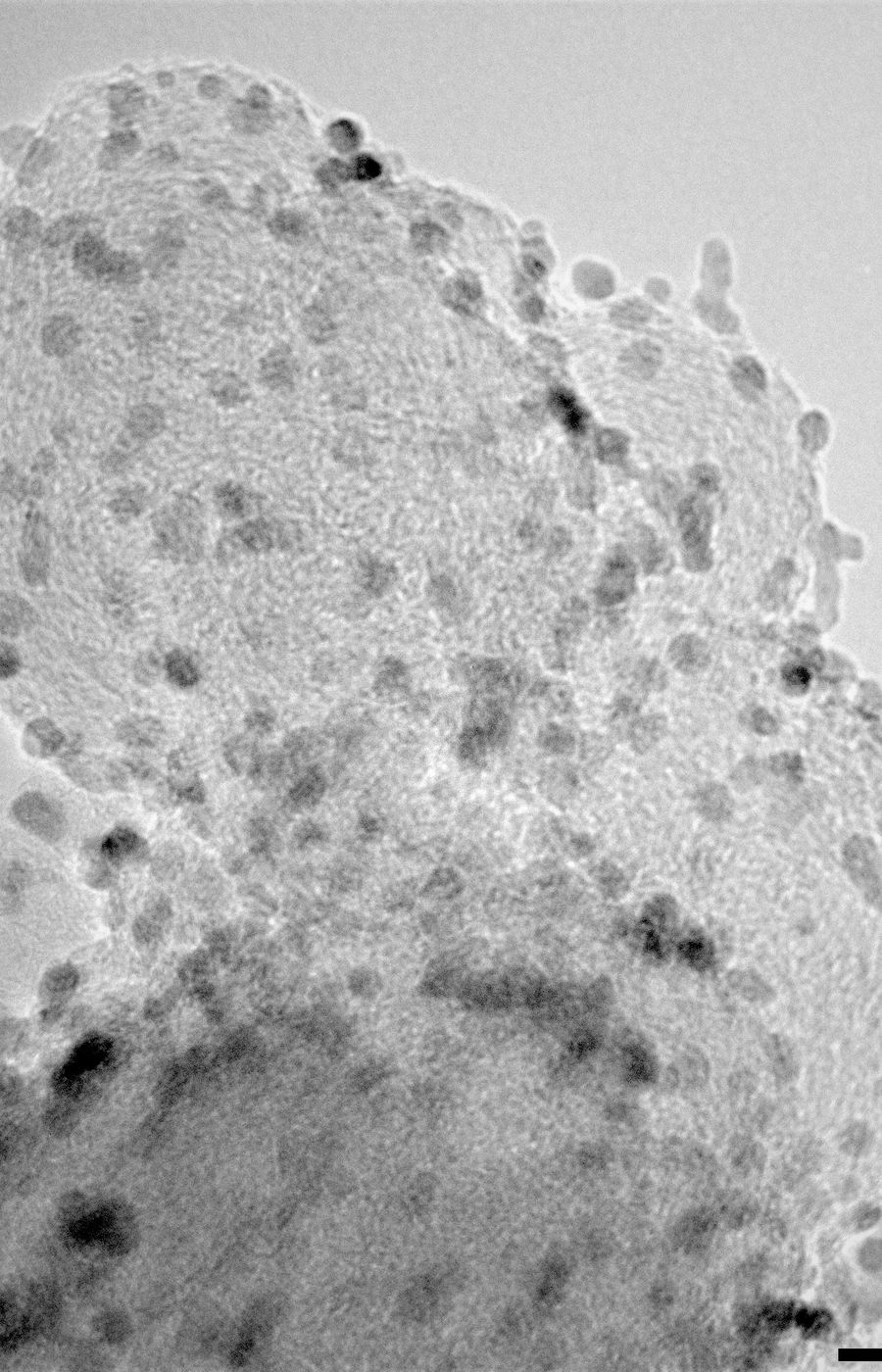

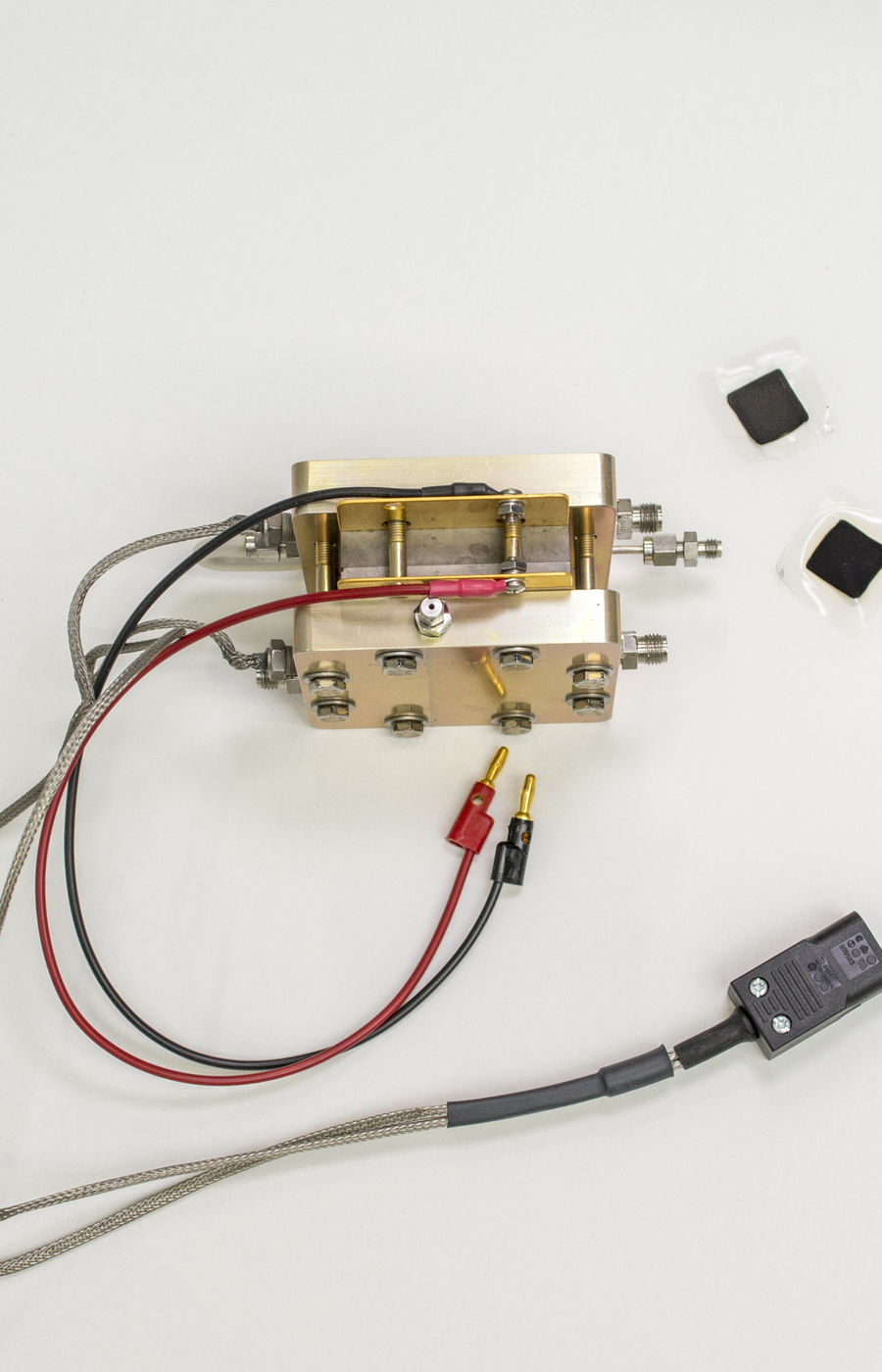
Researchers: Tanja Kallio, Associate professor, Moritz Rosenthal, Benjin Jin and Lilian Moumaneix, Electrochemical energy conversion and storage, Department of Chemistry and Materials Science, School of Chemical Engineering, Aalto University
Text: Peter Taggart

World-class materials research

Finnish runway to hydrogen business